Cancer-causing chemical benzene found in Johnson & Johnson sunscreen products
07/18/2021 / By Arsenio Toledo

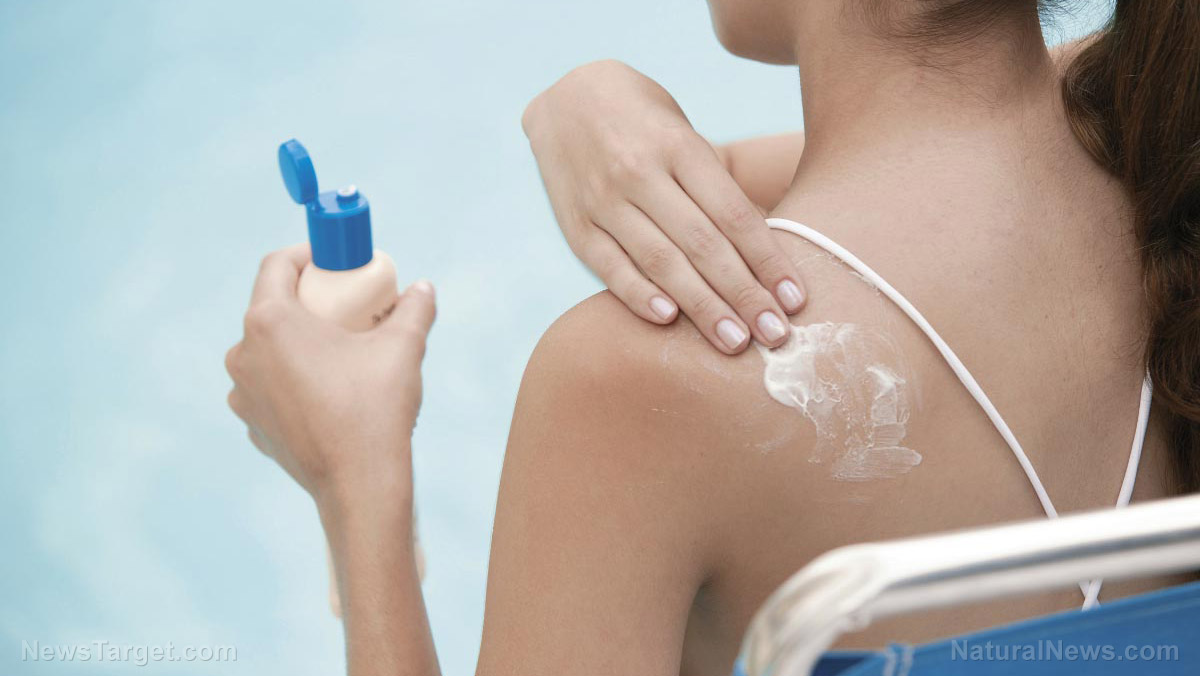
Johnson & Johnson announced on Wednesday, July 14, that it is recalling several of its sunscreen products from five different product lines after discovering they contained benzene, a known cancer-causing chemical.
The specific products being recalled are:
- Aveeno Protect + Refresh aerosol sunscreen.
- Neutrogena Beach Defense aerosol sunscreen.
- Neutrogena Cool Dry Sport aerosol sunscreen.
- Neutrogena Invisible Daily Defense aerosol sunscreen.
- Neutrogena Ultra Sheer aerosol sunscreen.
All the affected products are packaged in aerosols cans. All can sizes and all levels of sun protection factor, or SPF, were recalled.
Johnson & Johnson has recommended that consumers stop using the above-mentioned aerosolized sunscreens. The company has also already notified all of its distributors and retailers to stop selling the products. It is currently working on a way to get all of the unsold cans returned.
“Daily exposure to benzene in these aerosol sunscreen products at the levels detected in our testing would not be expected to cause adverse health consequences,” said Johnson & Johnson in its statement. “Out of an abundance of caution, we are recalling all lots of these specific aerosol sunscreen products. (Related: Cancer-causing chemical detected in 78 sunscreens, FDA petitioned to recall contaminated products.)
Benzene has strong links to blood cancers
Benzene is a widely used industrial chemical and a known carcinogen. Johnson & Johnson claimed it did not list benzene on the product labels because it is not used as an ingredient in sunscreen formulations. The company added that the cancer-causing substance may have contaminated the sunscreen products somewhere during the manufacturing process.
Significant traces of benzene were detected in dozens of popular sunscreen and similar products according to tests done by the online pharmacy company Valisure. Benzene was found in 78 of the nearly 300 sunscreen products Valisure tested, including products sold by big pharmacy retail companies like CVS.
Valisure petitioned the Food and Drug Administration (FDA) to get these contaminated products recalled. Johnson & Johnson withdrew its own products voluntarily. CVS has also recalled two of its own sunscreen products, After Sun Aloe Vera and After Sun Aloe Vera Spray.
David Light, founder and chief executive officer of Valisure, gave Johnson & Johnson a way out when he said the issue may be due to manufacturing contamination affecting only certain batches of the sunscreen products. The source of the contamination is still unknown, but Light has already urged manufacturers and consumers to take the possibility of benzene contamination seriously.
“Benzene is one of the most studied and concerning human carcinogens known to science. Its association with forming blood cancers in humans has been shown in numerous studies at trace levels of parts per million and below,” said Light in his company’s statement regarding the tests. ” The presence of this known human carcinogen in products widely recommended for the prevention of skin cancer and that are regularly used by adults and children is very troubling.”
The Centers for Disease Control and Prevention has confirmed that there are significant links between long-term benzene exposure and leukemia and other blood cancers.
In addition to being a dangerous carcinogen, benzene can damage the immune system and prevent cells from functioning properly. Symptoms of short-term exposure depend on whether a person inhales it, ingests it or gets it on skin and clothing. Symptoms can range from dizziness, irregular heart rate, convulsions and, at very high levels, death.
FDA called to enforce better standards to prevent carcinogens in consumer products
Over the past few months, concerning contaminants like benzene have been found in many personal care products like sunscreens and even hand sanitizers.
The Environmental Working Group (EWG), an environmental activist organization, has demanded that the FDA enforce its standards with regards to keeping hazardous chemicals out of everyday products “so that consumers don’t need to rely on independent testing from labs like Valisure,” said David Andrews, an EWG senior scientist.
The EWG has recommended that consumers avoid all spray and power-based sunscreen products. The group argues that consumers looking to prevent skin cancer should instead use lotion-based sunscreens. If sunscreen sprays are used it poses the risk of inhalation. Spray-based sunscreen products might also be inadequate.
In 2019, the FDA began recommending more safety tests on all spray and power-based sunscreen products to ensure that they pose minimal risk to the lungs if inhaled. In pilot tests, the FDA tested 14 sprays and found three did not meet its proposed standard. The agency refused to say which products to avoid.
“We expect the FDA to finalize its new sunscreens rules this fall,” said EWG senior vice president of government affairs Scott Faber. “We hope the agency sets deadlines for companies to test their sunscreens for contaminants like benzene.”
Head over to CancerCauses.news to learn more about the many different products in the market today that contain carcinogens like benzene.
Sources include:
Tagged Under: benzene, blood cancer, chemicals, consumer safety, Cosmetics, Environmental Working Group, ingredients, Johnson & Johnson, leukemia, personal care, products, sunscreen, toxic chemicals, toxic ingredients, toxic products
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 COSMETICS NEWS